Red Cabbage Color Indicator Chart
Red Cabbage Color Indicator Chart - Ph more than 7 = base. Is a substance that changes colour when it is added to acidic or alkaline solutions. Web in this video, education coordinator rosie demonstrates how you can make red cabbage indicator, a dark purple liquid which changes colour when it comes into contact with an acid or an alkali, and use it to create rainbows. Web chart of common ph indicators. Web red cabbage juice contains a natural ph indicator that changes colors according to the acidity of the solution. Web changing the colour of red cabbage. Web in this activity you will use red cabbage to make what is called an indicator solution. Various colouring materials in plants can act as indicators. Use the indicator chart to determine the ph. Web very low (under $20) safety. In this case adding something acidic (like lemon juice) will change it to one color while adding something basic (like bleach) will change it to another. Ph more than 7 = base. Red cabbage juice indicators are easy to make, exhibit a wide range of colors, and can be used to make your own ph paper strips. You can prepare. Each carbon has 4 bonds. Make your own ph indicator and use it to test the ph of various household solutions. In this case adding something acidic (like lemon juice) will change it to one color while adding something basic (like bleach) will change it to another. Web unlike most other natural ph indicators, cabbage juice displays a wide range. Web chart of common ph indicators. Requires adult supervision—some household solutions can be poisonous when mixed together or swallowed. Since red cabbage can be changed in color so easily, it's a great vegetable to use in a real scientific experiment on ph! It can tell you whether something is an acid or a base, as well as how acidic or. Each test tube contains a solution of red cabbage juice in water, but the ph of the solutions varies from ph = 2.0 (far left) to ph = 11.0 (far right). How to make a red cabbage ph indicator. The structures of the anthocyanin pigments which give the red cabbage its colour are subtly changed at varying ph. Yellow green. Set up jars or clear glasses and half fill with lemon juice, vinegar, water, bicarb soda and soapy water. It will remain purple or blue if the test solution is neutral. O liquid cleaning products (don’t use bleach) o solutions made by dissolving a solid such as baking soda, detergent, or baking powder in water. O fruit juice, for example:. Each test tube contains a solution of red cabbage juice in water, but the ph of the solutions varies from ph = 2.0 (far left) to ph = 11.0 (far right). Leave to cool for about 30 minutes. In this practical, students make an indicator from red cabbage. 15 minutes plus drying time. Web red cabbage juice contains a natural. Each test tube contains a solution of red cabbage juice in water, but the ph of the solutions varies from ph = 2.0 (far left) to ph = 11.0 (far right). If you use a blender, add the minimum amount of water you need. Red cabbage that is often found in homes can be used to prepare a solution that. Use red cabbage to create a ph indicator. Indicator solutions can change colors depending on what you add to them. Web a ph indicator is a substance which has one colour when added to an acidic solution and a different colour when added to an alkaline solution. O liquid cleaning products (don’t use bleach) o solutions made by dissolving a. Is a substance that changes colour when it is added to acidic or alkaline solutions. Use red cabbage to create a ph indicator. Indicator solutions can change colors depending on what you add to them. The indicator turns red when exposed to very acidic solutions, purple when. Since red cabbage can be changed in color so easily, it's a great. Requires adult supervision—some household solutions can be poisonous when mixed together or swallowed. Place the chopped cabbage into the pan and cover it with water. Web your red cabbage indicator should be dark blue in color. The structures of the anthocyanin pigments which give the red cabbage its colour are subtly changed at varying ph. The experiment is in two. The structures of the anthocyanin pigments which give the red cabbage its colour are subtly changed at varying ph. By anne marie helmenstine, ph.d. Use red cabbage to create a ph indicator. Web unlike most other natural ph indicators, cabbage juice displays a wide range of colors. Flavin is the pigment in red cabbage that produces the red colour shift (an anthocyanin). Web very low (under $20) safety. 15 minutes plus drying time. Web in this video, education coordinator rosie demonstrates how you can make red cabbage indicator, a dark purple liquid which changes colour when it comes into contact with an acid or an alkali, and use it to create rainbows. Place the chopped cabbage into the pan and cover it with water. The indicator turns red when exposed to very acidic solutions, purple when. How to make a red cabbage ph indicator. Web red cabbage juice contains a natural ph indicator that changes colors according to the acidity of the solution. Poor red cabbage juice into jug. Set up jars or clear glasses and half fill with lemon juice, vinegar, water, bicarb soda and soapy water. This simple activity can be set for learners to try at home with a responsible adult or used as a classroom experiment. Web in this activity you will use red cabbage to make what is called an indicator solution.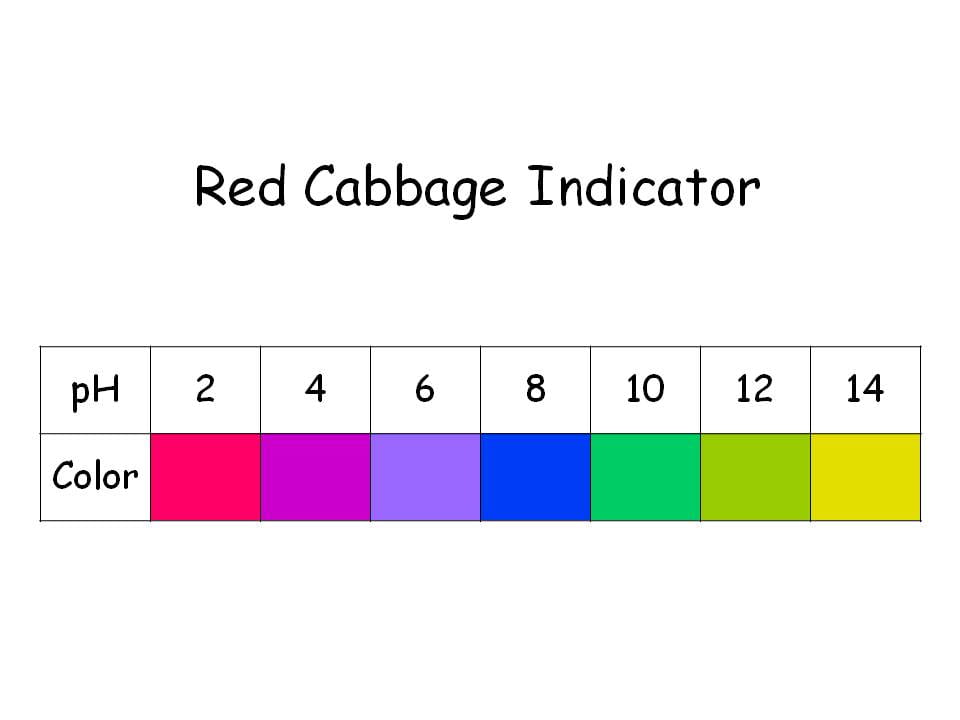
Red Cabbage Indicator Chemistry Demonstrations

Acids and Bases The Art of Science

Red cabbage dye (and pH indicator) ingridscience.ca

Red Cabbage Indicator Chemistry Demonstrations

Colorchange of (a) red cabbage anthocyanin and (b)... Download

Red cabbage dye (and pH indicator) ingridscience.ca

pH Indicator from Red Cabbage Red cabbage, Cabbage and Ph
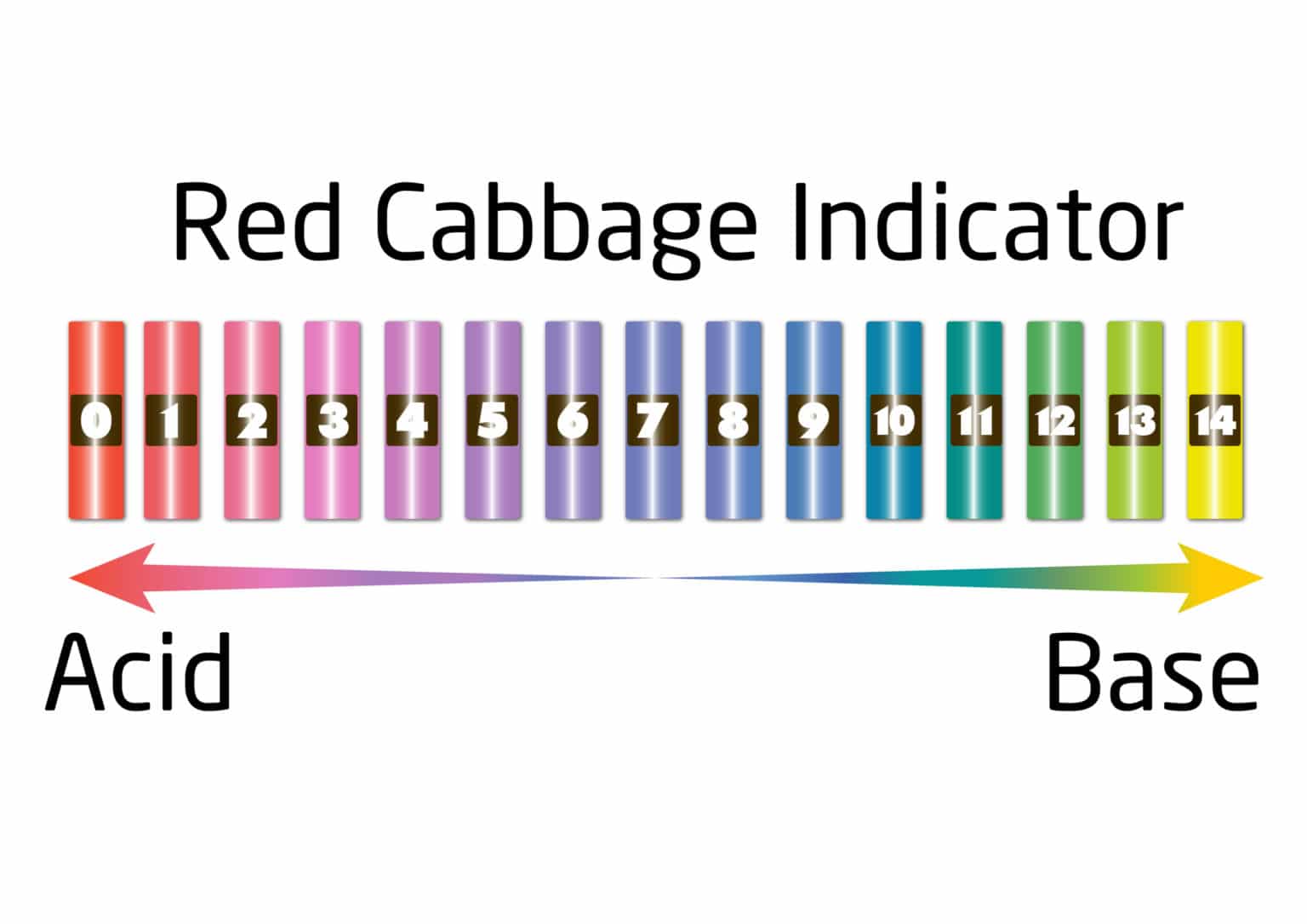
How to make a red cabbage pH indicator Chemistry for Kids

How to make a red cabbage pH indicator Chemistry for Kids Chemistry

Compound Interest Making a Red Cabbage pH Indicator The Method and
O Liquid Cleaning Products (Don’t Use Bleach) O Solutions Made By Dissolving A Solid Such As Baking Soda, Detergent, Or Baking Powder In Water.
Join Andrew And Samantha For Another Kitchen Science Experiment You Can Try At Home.
Pure Water Is Neutral, And So Is Petrol.
In This Practical, Students Make An Indicator From Red Cabbage.
Related Post: